Coexistence of Carbonyl and Ether Groups on Oxygen-Terminated (110)-Oriented Diamond Surfaces
Abstract
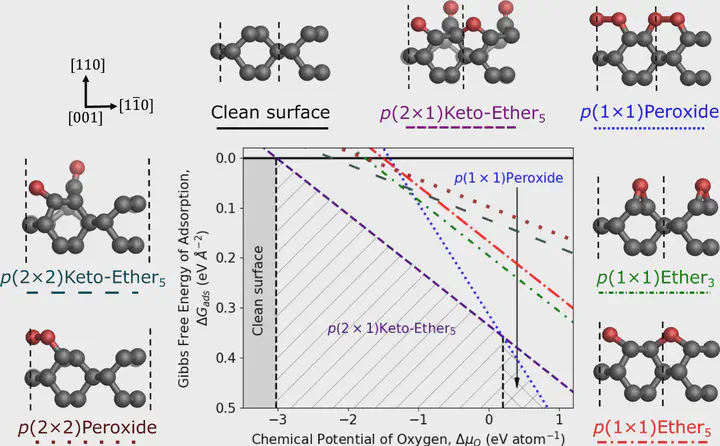
Diamond-based materials have unique properties that are exploited in many electrochemical, optical, thermal, and quantum applications. When grown via chemical vapor deposition (CVD), the growth rate of the (110) face is typically much faster than the other two dominant crystallographic orientations, (111) and (100). As such, achieving sufficiently large-area and high-quality (110)-oriented crystals is challenging and typically requires post-growth processing of the surface. Whilst CVD growth confers hydrogen terminations on the diamond surface, the majority of post-growth processing procedures render the surface oxygen-terminated, which in turn impacts the surface properties of the material. Here, we determine the oxygenation state of the (110) surface using a combination of density functional theory calculations and X-ray photoelectron spectroscopy experiments. We show that in the 0–1000 K temperature range, the phase diagram of the (110) surface is dominated by a highly stable phase of coexisting and adjacent carbonyl and ether groups, while the stability of peroxide groups increases at low temperatures and high pressures. We propose a mechanism for the formation of the hybrid carbonyl-ether phase and rationalize its high stability. We further corroborate our findings by comparing simulated core-level binding energies with experimental X-ray photoelectron spectroscopy data on the highest-quality (110)-oriented diamond crystal surface reported to date.